AstraZeneca vs B1351. However if the gap was 12 weeks or longer efficacy jumped to 813.

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet
One shot of the Pfizer option was 85 per cent effective at preventing hospitalisation at 28-34 days post-vaccination whereas one shot of the AstraZeneca vaccine was 94 per cent effective at preventing hospitalisation in the same interval.

Astrazeneca vaccine efficacy. Participants 156 930 adults aged 70 years and. Vaccines to prevent COVID-19 infection are crucial for an effective global pandemic response. Public Health Scotland University of Edinburgh study showed the PfizerBioNTech vaccine reduced hospitalisations by 85 and 94 for the Oxford AstraZeneca vaccine.
AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the. In comparison the effectiveness against the B117 was 661. While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people.
Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults aged 18 years and older. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. It is important to recognise that this is still early data but it is still highly encouraging.
Setting Community testing for covid-19 in England. So to get the best protection from the AstraZeneca vaccine you need at least 12 weeks between your first and second shot. The principal thrust of the article is to gauge efficacy against the B1351 virus variant now prevalent in South Africa.
Theres a new journal article at the New England Journal of Medicine detailing a Phase 1b2 clinical trial of the AstraZeneca COVID-19 vaccine conducted in South Africa. The AstraZeneca vaccine - which was administered to its first patients in the UK on January 4 - was 62 percent effective in an Oxford trial based on recipients having two doses of the vaccine. What does Vaccine Efficacy actually mean.
In the new study 1054 individuals were tested for the B16172 variant with 244 having received the AstraZeneca vaccine. The Lancet states that the efficacy for a delay of 6 weeks or less between doses is 551 but with 12 weeks or over is 813. Does it work against new variants.
The effectiveness of the AZ vaccine two-dose regimen versus this variant was 598. Objective To estimate the real world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against confirmed covid-19 symptoms including the UK variant of concern B117 admissions to hospital and deaths. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern.
1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. Design Test negative case-control study. The headline figures for Vaccine Efficcacy released over the.
In The Lancet Merryn Voysey and colleagues1 report the updated primary efficacy results for the OxfordAstraZeneca ChAdOx1 nCoV-19 AZD1222 vaccine from three single-blind randomised controlled trials in the UK and Brazil and one double-blind study in South Africa24 The ChAdOx1. Studies of the efficacy of this vaccine have been wide-ranging. The news isnot good.
A pooled analysis of four large studies found that in groups that received two standard. New vaccine efficacy results are reported now in The Lancet. Another study cited 549 and 824.
Note that the efficacy of the AstraZeneca vaccine was never tested on the 56 age cohort and. According to AstraZeneca the vaccine maintains 76 efficacy after the first dose allowing for a longer interval of 12 weeks or more for the second dose. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection.
Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. I think people should be reassured that the AstraZeneca vaccine is excellent Professor Drummer said. COVID-19 vaccines made by AstraZeneca AZNL and the Pfizer-BioNTech PFEN 22UAyDE alliance remain broadly effective against Delta.

Oxford Astrazeneca Study Supports Uk Decision To Delay Second Doses Financial Times

Pfizer Astrazeneca Vaccines Provide Similar Protection Against Symptomatic Covid 19 Study Science News

The Best Vaccine For You Is The One Available To You Right Now

Astrazeneca Says Shot 90 Effective

Explainer The Confusion Of Covishield Dosing
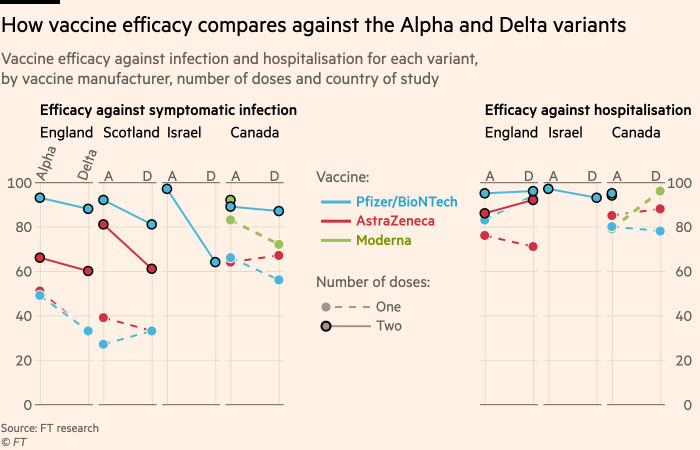
How Effective Are Coronavirus Vaccines Against The Delta Variant Financial Times
.jpg)
Uk Public Health Authorities Review Early Data On The Effectiveness Of Pfizer And Astrazeneca Covid 19 Vaccines

Worldimmunizationweek 2021 Everything You Need To Know About Covid 19 Vaccines Speaking Of Research

Germany Restricts Use Of Astrazeneca Vaccine To Over 60s In Most Cases News Dw 30 03 2021
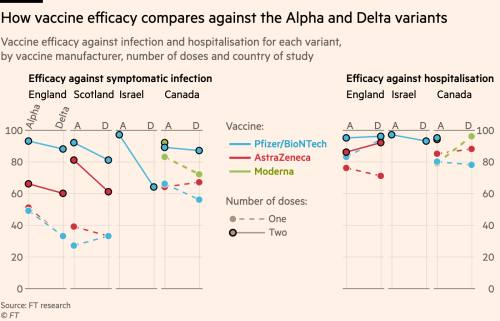
How Effective Are Coronavirus Vaccines Against The Delta Variant Financial Times
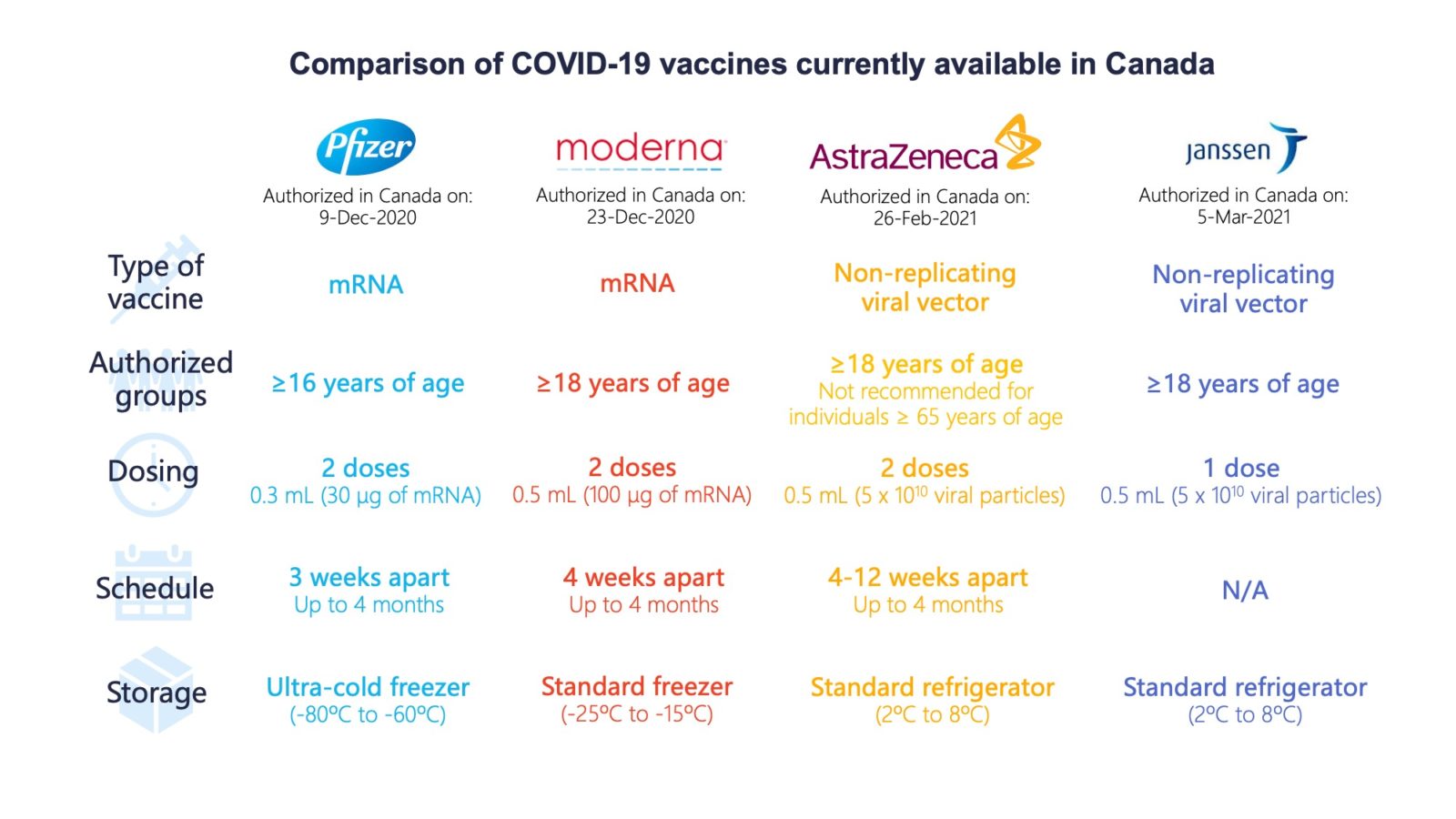
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
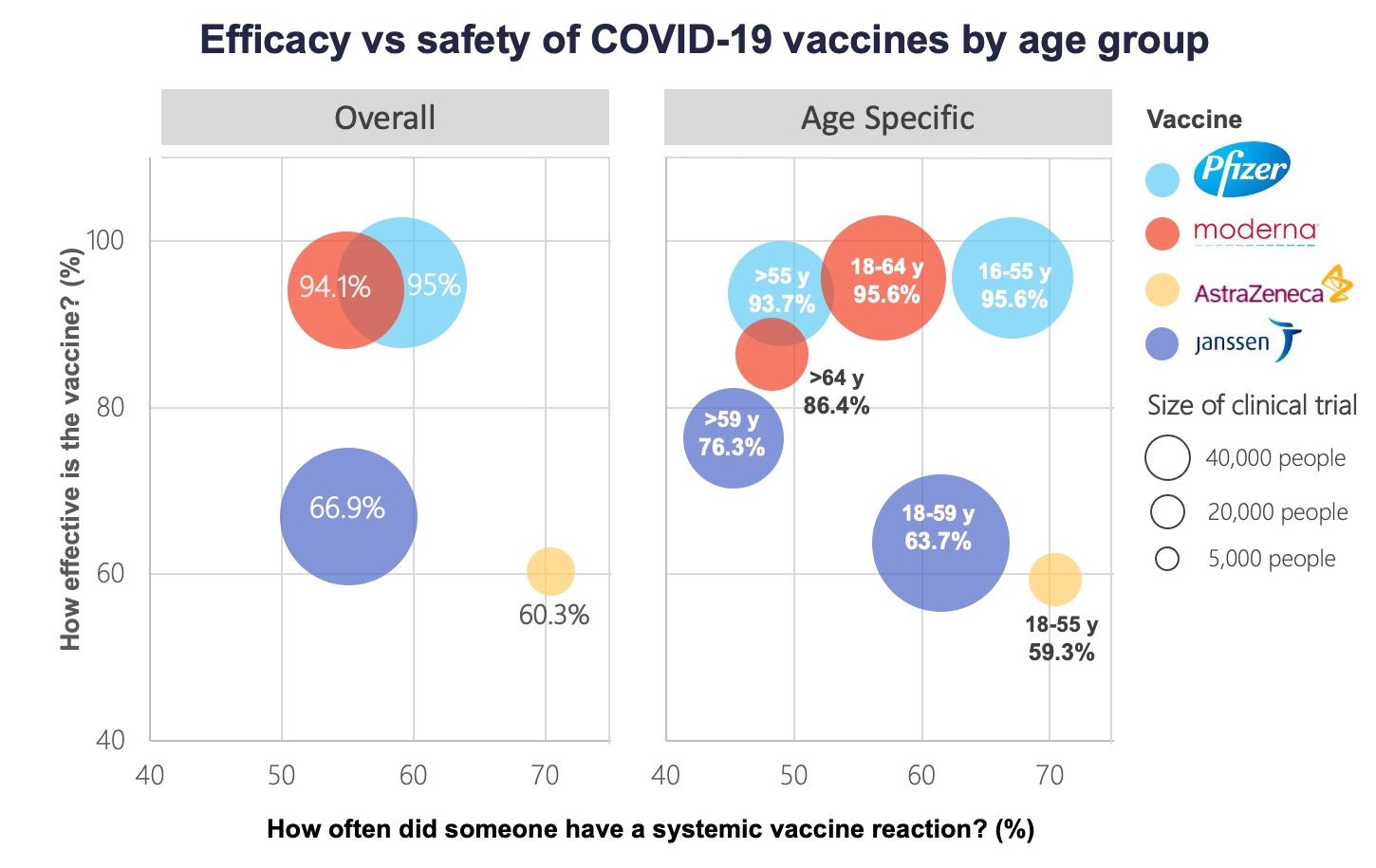
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
Coronavirus Vaccine Efficacy Compared To Shots For Other Viruses
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate

Disuntik Sinovac 99 49 Persen Kebal Covid 19 Astrazeneca 97 26 Persen
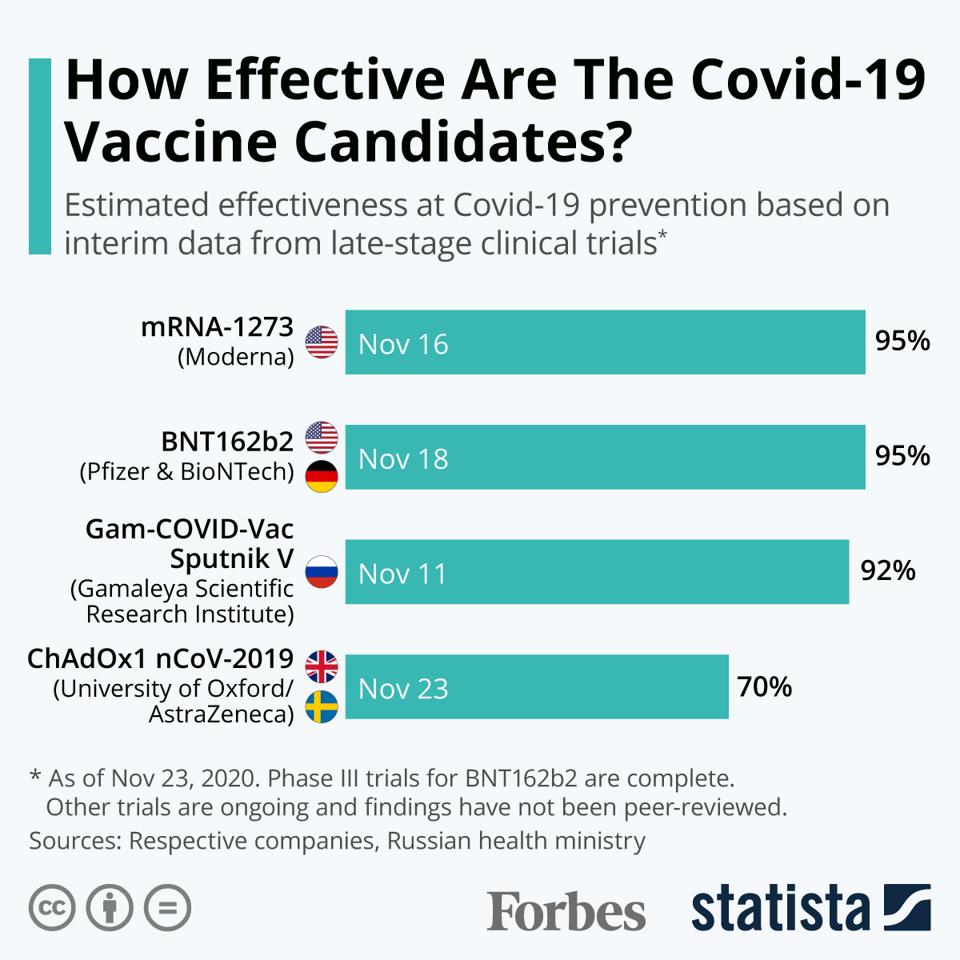
How Effective Are The Covid 19 Vaccine Candidates Infographic
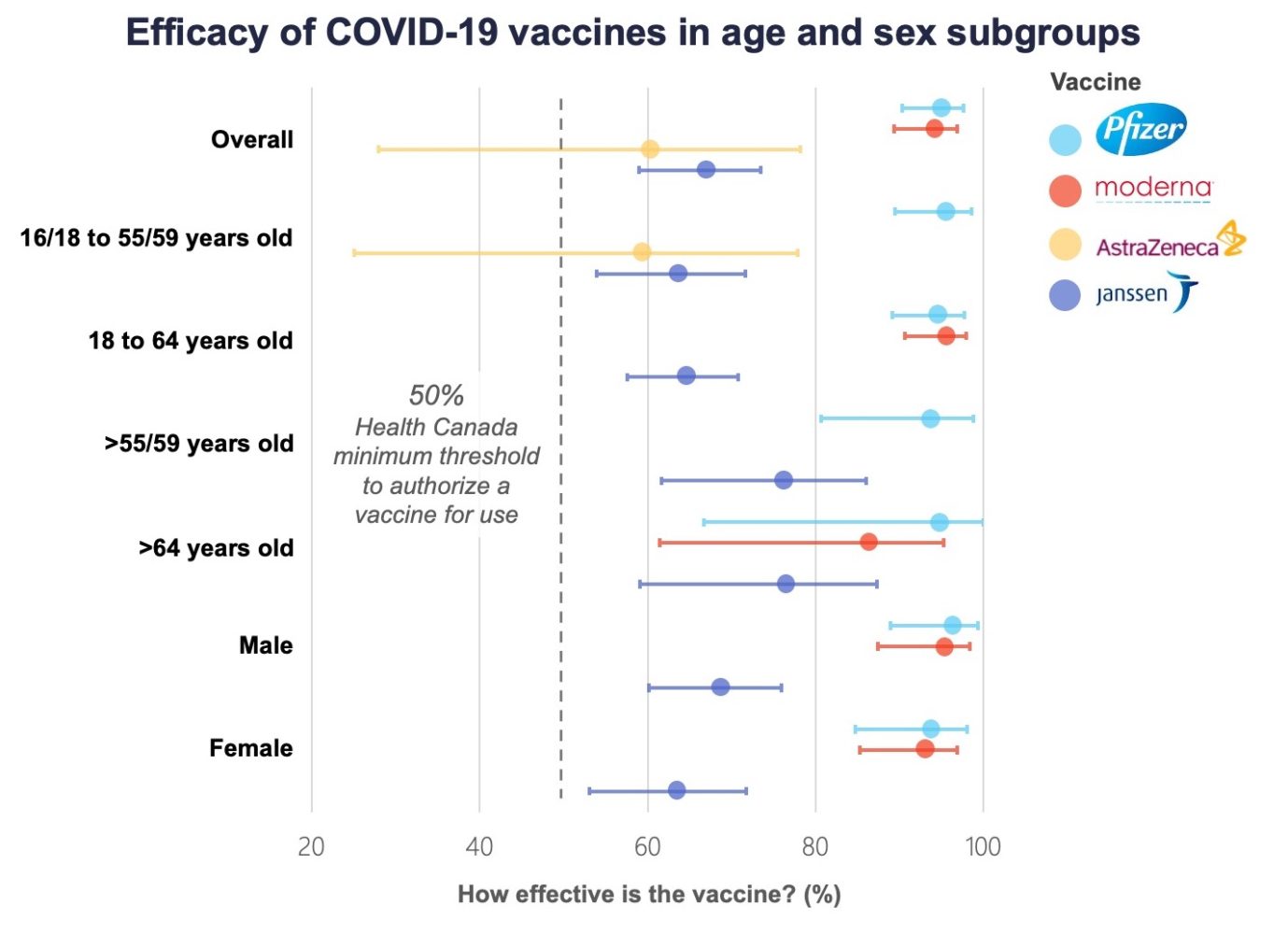
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
YOU MAY LIKE :
