AstraZeneca vaccine tribulations The fuller data still revealed a higher efficacy rate than in the trial conducted outside the US which was used to win regulatory approval in the UK and the EU. We apply the average B1351P1D614GB117 ratio for Novavax and Johnson Johnson to determine the vaccine-specific efficacy at preventing disease.
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
Vaccine efficacy for the prespecified primary analysis combining dose groups against the primary endpoint of COVID-19 occurring more than 14 days after the second dose was 704 958 CI 548 to 806.

Astrazeneca vaccine efficacy rate. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. 30 05 of 5807 participants in the ChAdOx1 nCoV-19 group vs 101 17 of 5829 participants in the control group. At this point only vaccine efficacy VE data is available since effectiveness depends on many different factors and requires a longer observation period.
A study published in April 2021 by researchers from the COVID-19 Genomics United Kingdom Consortium the AMPHEUS Project and the Oxford COVID-19 Vaccine Trial Group indicated the OxfordAstraZeneca vaccine showed somewhat reduced efficacy against infection with the Alpha variant lineage B117 with 704 efficacy in absolute terms against Alpha versus 815 against. On March 25 2021 AstraZeneca released primary analysis that the vaccine demonstrated 76 efficacy against symptomatic COVID-19 100 efficacy against severe or critical disease and hospitalizations and 85 efficacy against symptomatic COVID-19 in people 65 years and older. One shot of the Pfizer option was 85 per cent effective at preventing hospitalisation at 28-34 days post-vaccination whereas one shot of the AstraZeneca vaccine was 94 per cent effective at.
704 95 CI 548 to 806 overall VE against symptomatic COVID-19 14 days after 2nd dose. Compared to other vaccines the Pfizer vaccine has 95 efficacy Sinovac vaccine has 504 9125 efficacy and CanSinoBIO vaccine has 657 efficacy. Interim analysis of a phase III trial has found that the vaccine produced by Oxford University and AstraZeneca was 79 effective at preventing symptomatic covid-19 and 100 effective at preventing severe disease and admission to hospital.
Among the 32 449 participants randomised 21 to vaccine. According to AstraZeneca the vaccine maintains 76 efficacy after the first dose allowing for a longer interval of 12 weeks or more for the second dose to be administered which then increases. The study also demonstrated that the vaccines are around 65 effective more than 21 days after just one dose of either vaccine and that those who do get infected have milder symptoms.
AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the. Trial slightly lower than the level announced. AstraZeneca COVID-19 Vaccine ChAdOx1-S recombinant.
AstraZeneca said its COVID-19 vaccine had a 76 per cent efficacy rate at preventing symptomatic illness in a new analysis of its major US. The results announced in an AstraZeneca press release have yet to be formally peer reviewed. According to The University of Oxford and AstraZeneca interim results from phase 3 clinical trial the vaccine had an efficacy of 79 per cent against symptomatic Covid-19.
While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people who received two full doses and closer to 90 percent in. AstraZeneca trials in the US reveal that out of 32449 participants the efficacy rate against Covid-19 was 76 while for those aged above 65 the rate was 85. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern.
So that means that potentially theyre less likely to pass that virus on to people around them Dr Quinn said. All other vaccines without available data. Does it work against new variants.
Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. We find the ratio of the efficacy between B1351 and B117 as observed in the Qatar study. The AstraZeneca vaccine - which was administered to its first patients in the UK on January 4 - was 62 percent effective in an Oxford trial based on recipients having two doses of the vaccine.
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
What To Make Of Covid 19 Vaccine Efficacy Rates Facebook

Explainer The Confusion Of Covishield Dosing
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
How Effective Are Coronavirus Vaccines Against The Delta Variant Financial Times

Astrazeneca Says Shot 90 Effective
Coronavirus Vaccine Efficacy Compared To Shots For Other Viruses
Astrazeneca Oxford Defend Vaccine Trials After Questions Raised In Us

Germany Restricts Use Of Astrazeneca Vaccine To Over 60s In Most Cases News Dw 30 03 2021
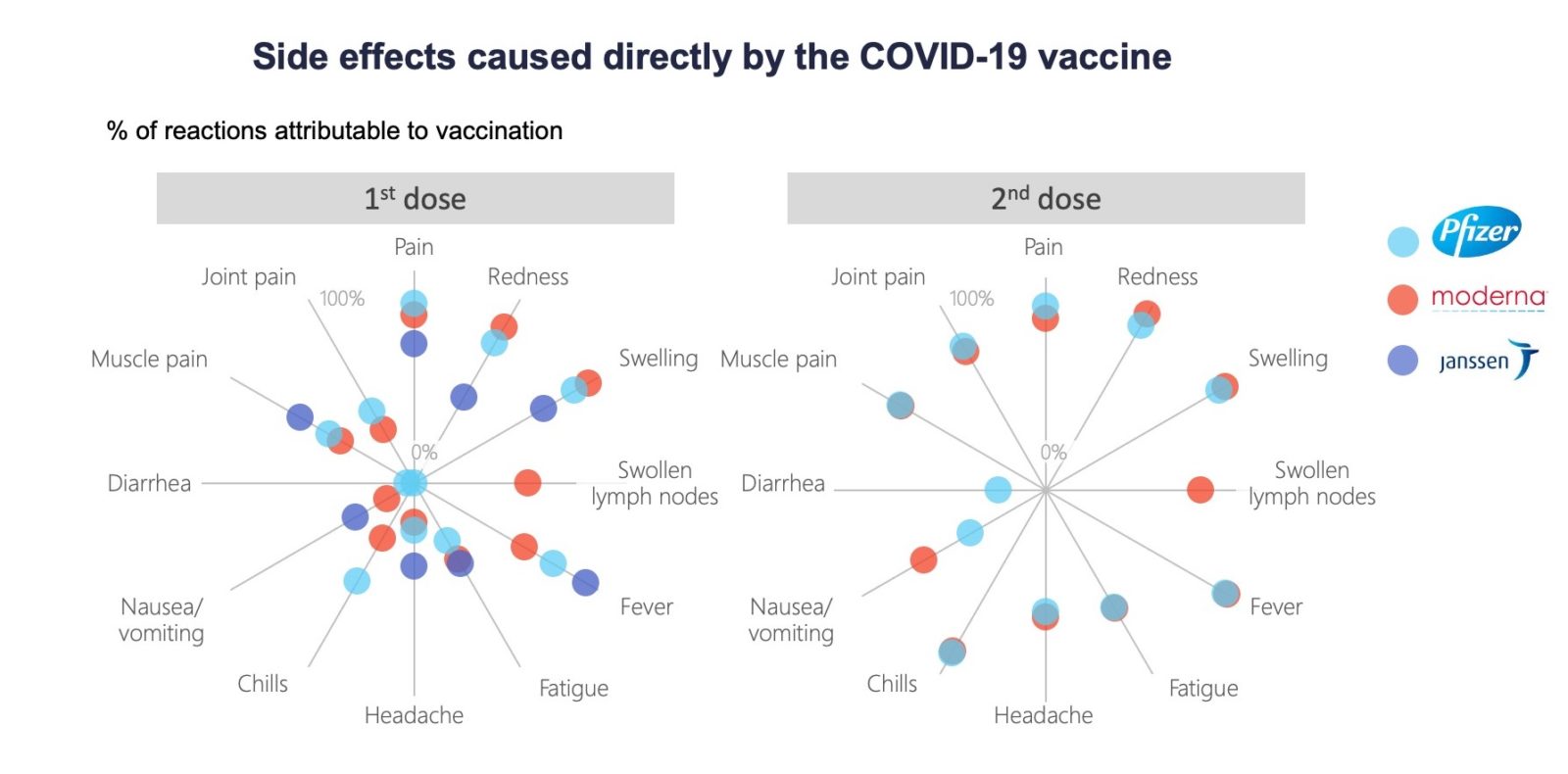
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
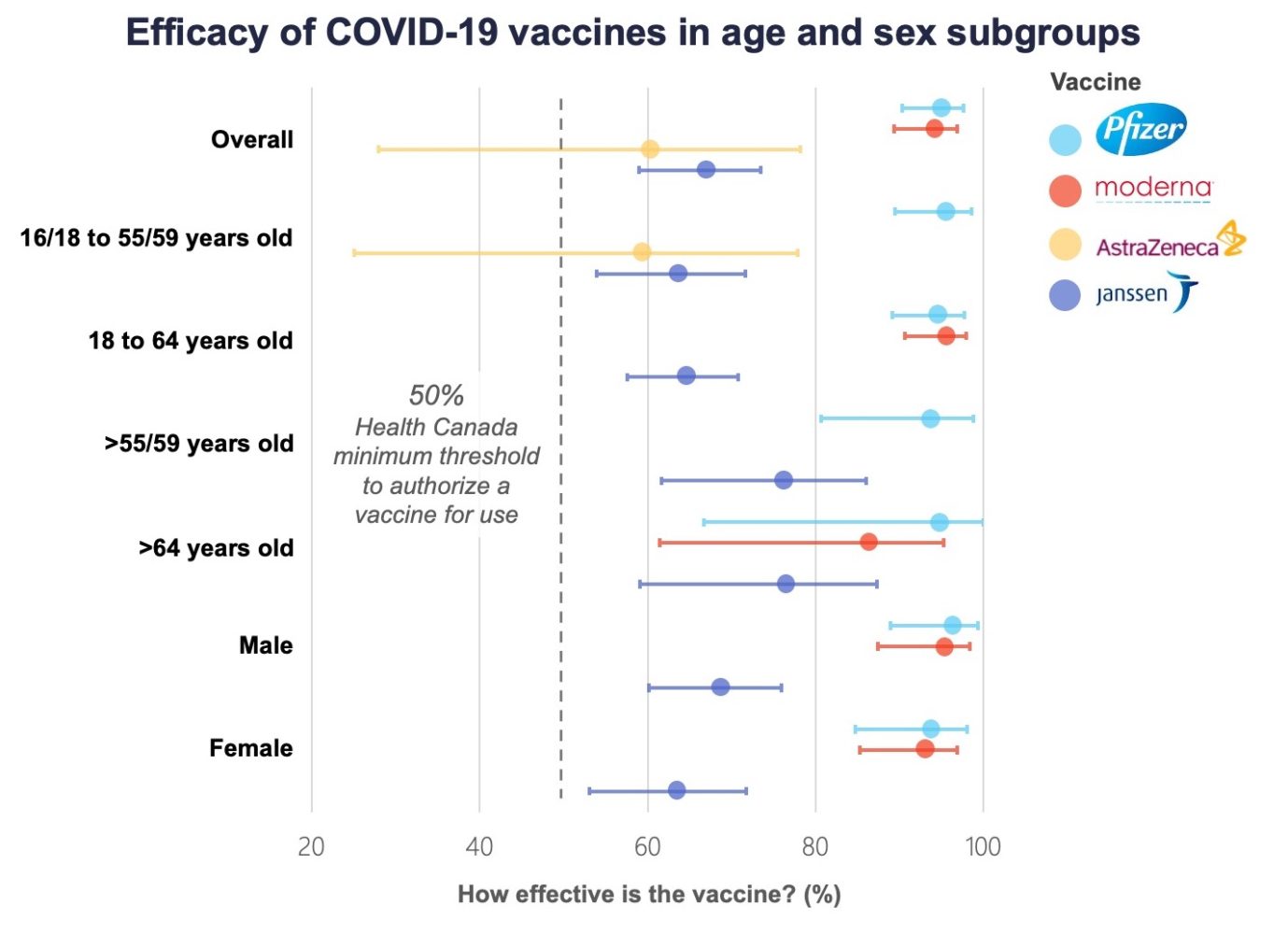
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
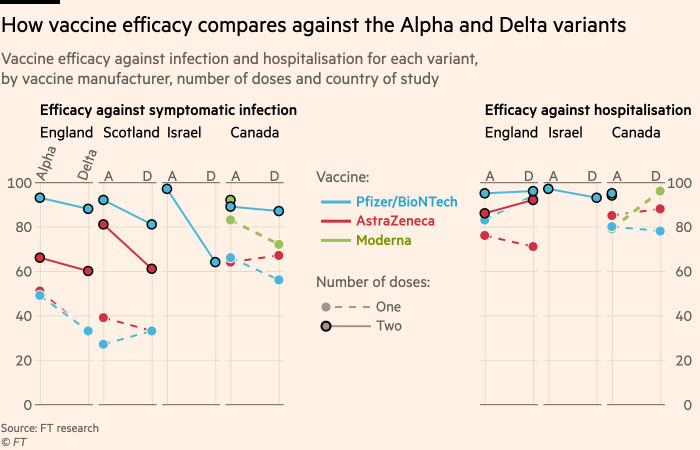
How Effective Are Coronavirus Vaccines Against The Delta Variant Financial Times
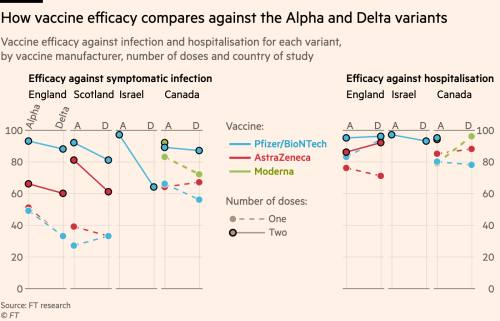
How Effective Are Coronavirus Vaccines Against The Delta Variant Financial Times

Pfizer Vs Moderna Vs Astrazeneca Whose Efficacy To Trust Businesstoday

Oxford Astrazeneca Study Supports Uk Decision To Delay Second Doses Financial Times

How Oxford Covid Vaccine Works And How It Differs From The Pfizer Moderna Shots Coronavirus Outbreak News
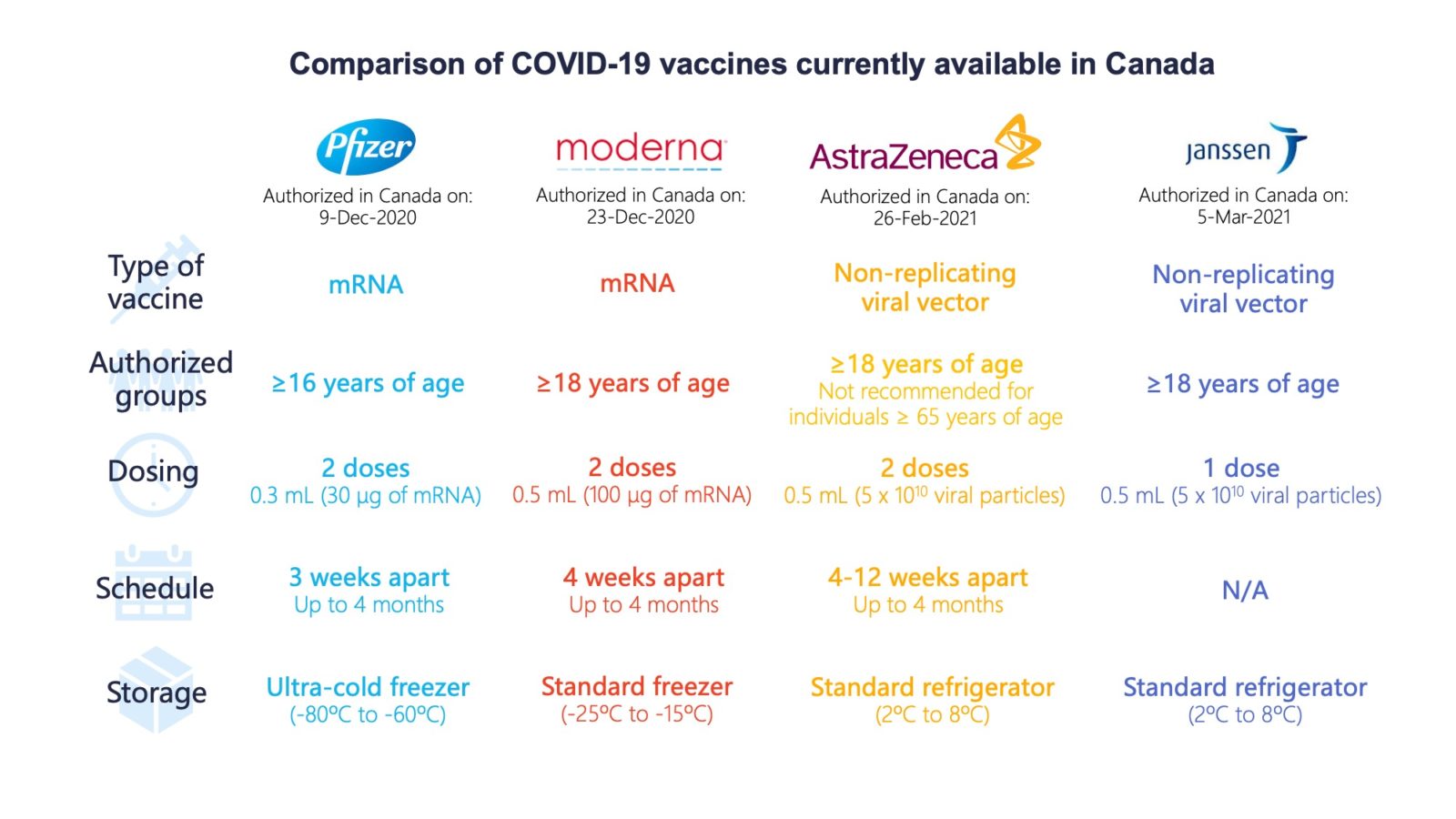
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate

Pfizer Astrazeneca Vaccines Provide Similar Protection Against Symptomatic Covid 19 Study Science News

Vaccine Study Finds Jabs Effective
YOU MAY LIKE :